a. molecules take up three-dimensional space, obviously, and
b. that electrons repel one another.
The result is a series of diagrams which occur, given a certain number of bonded elements as well as unbonded pairs of electrons...
Such special geometric molecules can be specially named:
Where
A represents the central atom,
X# represents the number of outer bonded atoms,
and
E# represents the number of lone electron pairs.
Here is a table of different geometries of different chemical compounds you ought to know for our test!
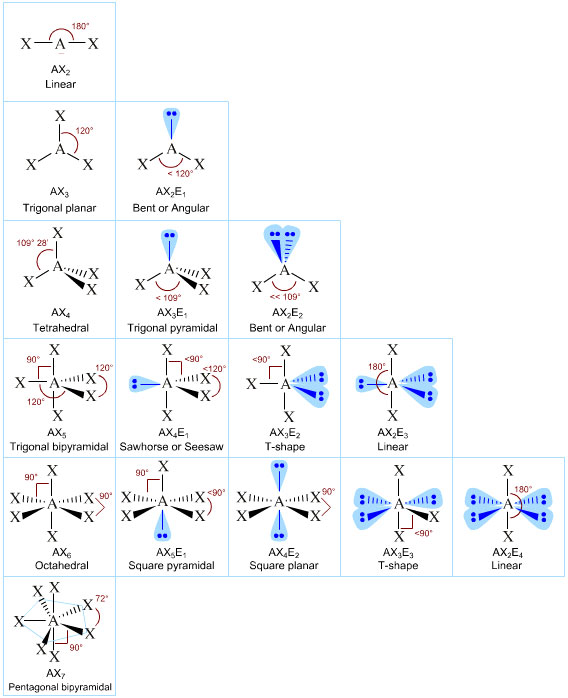
If you find yourself, daring to ask why they form these interesting shapes. Don't worry, the answer is simple. Because electrons repel each other in 3D space, each bonded or lone pair of electrons will simultaneously try to space themselves out to create the least amount of repulsion possible.
Watch the video if
a. you're extremely bored.
b. VSEPR Theory still confuddles you.
c. you want to watch a video that concisely demonstrates VSEPR in a way words and pictures cannot.
No comments:
Post a Comment